Since the introduction of antibiotics into human medicine in the 1940s, resistance against antibiotics has emerged at an alarming rate and is now a major threat to public health. The problem in chronic wounds is that bacteria form biofilms. These biofilms protect the bacteria from antimicrobials (e.g. antibiotics); this is called biofilm tolerance (Ref 1). In this blog we would like to address the challenges around biofilm tolerance in chronic wounds.
The formation of biofilms
All open wounds contain bacteria. Chronic wounds are open wounds that do not heal, for instance a diabetic foot wound or open leg (ulcus cruris) wound e.g. due to venous insufficiency. In the early stages of the formation of a chronic wound, these free-floating (planktonic) bacteria are generally destroyed by the host’s immune system. However, bacteria have been shown to have a preference to attach to each other, forming biofilms (see Figure 1). The bacteria in a biofilm form permanent attachments and produce an extracellular biofilm matrix. This structure offers extra protection from external influences (Ref 2). Two commonly known examples of a biofilm are dental plaque and the slimy layer in a dog’s food bowl.
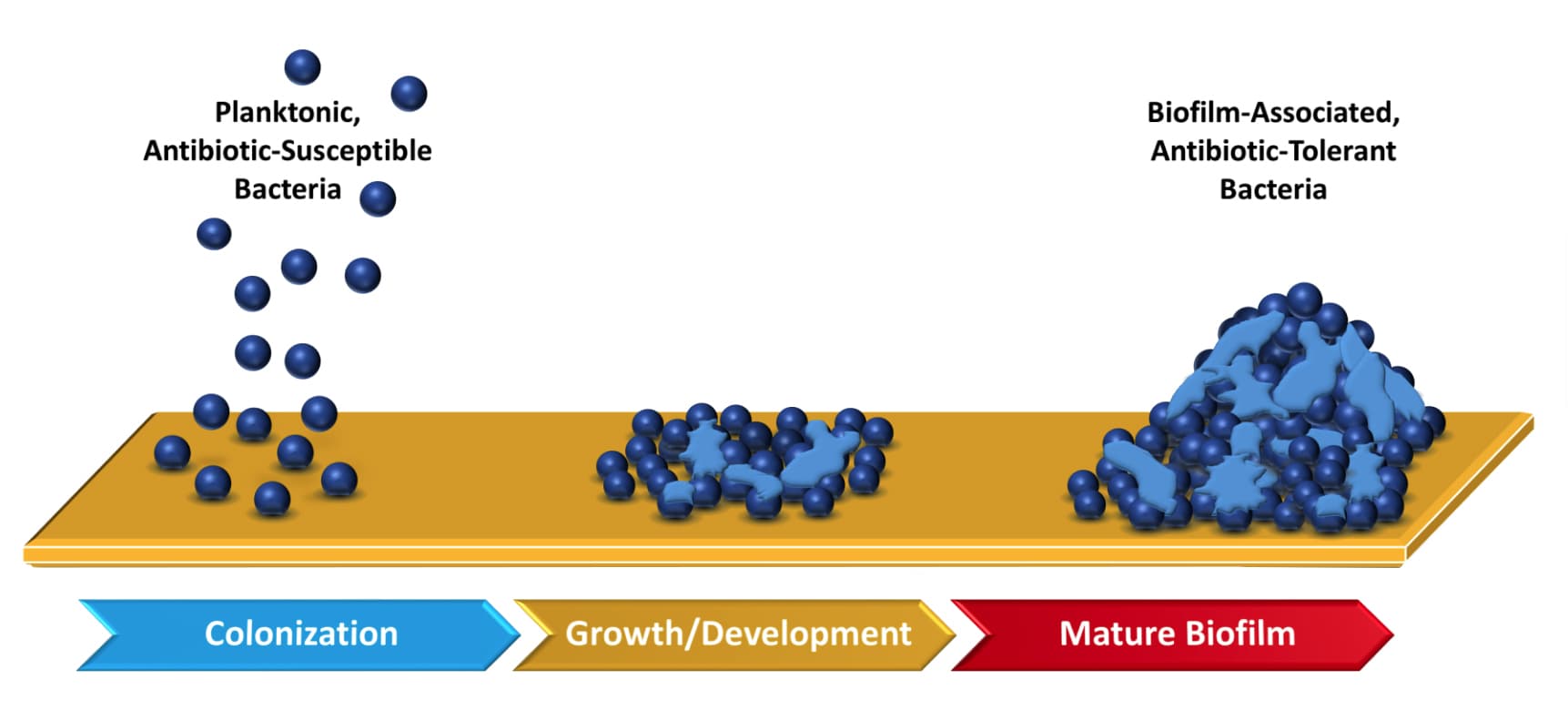
Figure 1: Illustration of biofilm formation from planktonic bacteria and subsequent biofilm maturation. Adapted from Ref 3.
Why biofilms cause problematic infections
Evidence demonstrates that a biofilm plays a significant role in the inability of chronic wounds to heal (Ref 5). Biofilms are present in only 6% of acute wounds but in over 90% of chronic wounds (Ref 5). Young biofilms are much more susceptible to antibiotics than mature biofilms. This underlines the importance of early interventions in the treatment of biofilm infections (Ref 6).
The primary reason why biofilms cause problematic infections is the ability to tolerate antibiotics and components of the immune system. The currently used antibiotics may decrease the number of bacteria in biofilms, but they cannot completely eradicate the biofilms. This results in relapses of biofilm infections (Ref 6), with as result: the wound does not close. On top of this, every biofilm has different characteristics so that a clinical approach must be tailored to the specifics of a given biofilm. This makes a biofilm hard to treat (Ref 5).
How to treat biofilms?
Unfortunately, many of the treatments currently available were designed to treat acute infections, which, unlike chronic infections, tend to appear quickly and run their course over a short period of time. Planktonic bacteria typically respond to antibiotics and are easily exterminated by a healthy immune system. Mature biofilms can develop in chronic wounds within 10 hours and persist indefinitely while the wound remains open. In addition, a clinical study found that although surgical debridement of chronic wounds effectively removed biofilm communities from the wound beds, biofilms began to reemerge 2 days after the initial debridement. High numbers of bacteria in the mature biofilms were identified 3 days after debridement. This indicates that there is a small window of opportunity during which the planktonic bacteria recolonising the open wound bed are susceptible to regular antimicrobial or other treatments (Ref 4).
Current therapies and therapies under development are often a combination therapy of debridement to remove the biofilm and an additional therapy to prevent the biofilm from coming back (Ref 7). As additional therapy antimicrobials or innovative strategies could be used. With the rise in antimicrobial resistance and biofilm tolerance, innovative strategies such as cold plasma (ionized gas) described in Ref 8, Ref 9 and Ref 10 could be of great help. Other innovative strategies are also described, see Ref 4.
Cold plasma for biofilm control
Numerous in vitro and in vivo studies already demonstrated the antimicrobial potential of cold plasma, not only against individual bacteria, but also against antibiotic-resistant bacteria as well as biofilms (Ref 11). It has been presumed that development of bacterial resistance to cold plasma is unlikely. Undoubtedly cold plasma has demonstrated potential for the control of biofilm related infections. Furthermore, it is shown that cold plasma can, besides its antibacterial effect, promote tissue regeneration (granulation tissue) and enhance local blood supply (Ref 8).
Conclusion: additional novel strategies could be of great help for treatment of chronic wounds
Fast recurrence of biofilm and tolerance to antibiotics is problematic for treatment of chronic wounds. Therefore, additional novel strategies such as cold plasma could be of great help. It is paramount that effective treatment is performed within 2 to 3 days after debridement, the time window before the biofilm reemerges.
Do you discriminate between wounds that contain planktonic bacteria or biofilms? If yes, what do you use to treat biofilms? Please post your reaction below.
A notification on new blogs will be posted in our newsletter. Below you can subscribe.
References:
1 Bowler P.G. (2018). Antibiotic resistance and biofilm tolerance. A combined threat in the treatment of chronic infections. Journal of wound care 27 (5):273-277
2 Collette H Thomson (2010). Biofilms: do they affect wound healing? International Wound Journal 8(1).
3 Aaron T. Garrison and Robert W. Huigens (2017). Eradicating Bacterial Biofilms with Natural Products and their Inspired Analogues that Operate Through Unique Mechanisms. Current Topics in Medicinal Chemistry 17(14): 1-8
4 Omar et al. (2017). Microbial Biofilms and Chronic Wounds. Microorganisms 5(9)
5 Attinger and Wolcott (2012). Clinically Addressing Biofilm in Chronic Wounds, Advances in wound care 1(3): 127-132
6 Ciofu and Tolker-Nielsen (2019). Tolerance and Resistance of Pseudomonas aeruginosa Biofilms to Antimicrobial agents – How P. aeruginosa Can Escape Antibiotics. Frontiers in Microbiology 10: Article 913
7 Metcalf D.G. and Bowler P.G. (2013). Biofilm delays wound healing: A review of the evidence. Burns & Trauma 1 (1): 5-12
8 Whitepaper. Stimulation of wound healing using cold plasma: https://www.plasmacure.nl/en/whitepaper/
9 Wiegand C. (2019). Potential of cold atmospheric pressure plasma (CAPP) in wound management. Wounds International 10 (4): 26-31
10 Metelmann H.R. et al. (2018). Comprehensive clinical plasma medicine: Cold Physical Plasma for Medical Application
11 Padrig B Flynn and Brendan F Gilmore (2018). Understanding plasma biofilm interactions for controlling infection and virulence, Journal of Physics D: Applied Physics, 51 263001
These articles have been interpreted by people from Plasmacure BV. Questions related to this blog are answered by our wound experts. Mail to blog@plasmacure.nl


React